
The GC-ECD also requires a make-up gas if helium is used as the carrier gas, since this make-up gas serves to provide the electrons that are not contained within the helium, as the base current. Oxygen and water impurities can also oxidise the radioactive nickel source used to generate the base current, additionally the carrier gas should have exceptionally low levels of halocarbons as the ECD is extremely sensitive to these compounds. These can interact with the stationary phase causing significant problems including high baseline noise and column bleed in the output gas chromatogram, resulting in reduced analyser sensitivity and decreased column lifespan. An ECD is often used in conjunction with a GC instrument.Īs common with other GC techniques a carrier gas is required with low water and oxygen impurities. This reduction is recorded as a positive peak in the detection of components. When a sample passes between these two electrodes, the molecules pick up some of the electrons, causing a reduction in the current. An ECD operates using two electrodes with a current passing between them.
EXAMPLE OF ELECTRON CAPTURE FULL
Some common radioisotopes that decay by electron capture include:įor a full list, see the Chart of the Nuclides.Electron Capture Detector (ECD) is a technique used to analyse halogenated compounds and is primarily found in the environmental, forensic and pharmaceutical markets. A good example of this effect would be silver, as its light isotopes use electron capture and the heavier ones decay by negative beta emission.
EXAMPLE OF ELECTRON CAPTURE HOW TO
The following example shows how to capture video from a desktop window. Īround the elements in the middle of the periodic table, isotopes that are lighter than stable isotopes of the same element tend to decay through electron capture, while isotopes heavier than the stable ones decay by (negative) beta decay. Access information about media sources that can be used to capture audio and.

There are four different types of emissions that occur. In most instances, the atom changes its identity to become a new element.

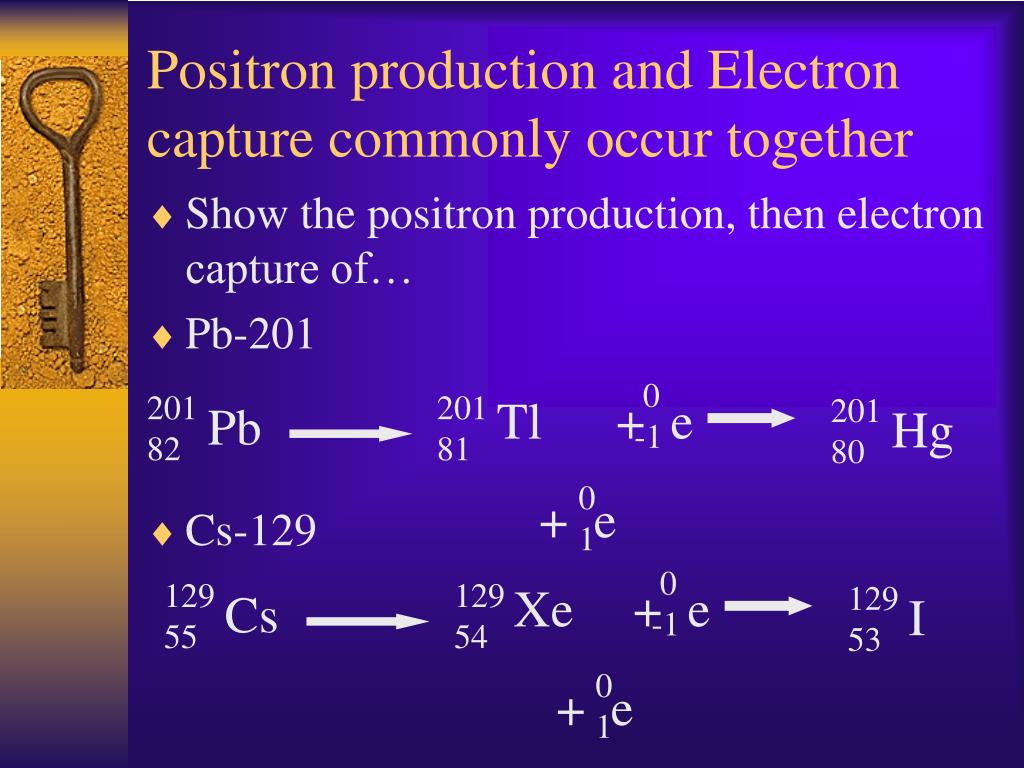
Nuclear Decay Processes Radioactive decay involves the emission of a particle and/or energy as one atom changes into another. Anomalies in elemental distributions are thought to be partly a result of this effect on electron capture.Ĭhemical bonds can also affect the rate of electron capture to a small degree (generally less than 1%) depending on the proximity of electrons to the nucleus. Example 11.2.1 shows how we can identify a nuclide by balancing the nuclear reaction. It is hypothesized that such elements, if formed by the r-process in exploding supernovae, are ejected fully ionized and so do not undergo radioactive decay as long as they do not encounter electrons in outer space. (Please note that it is one of the initial atom's own electrons that is captured, not a new, incoming electron as might be suggested by the way the above reactions are written.) Radioactive isotopes which decay by pure electron capture can, in theory, be inhibited from radioactive decay if they are fully ionized ("stripped" is sometimes used to describe such ions). When transiting to the ground state, the atom will emit an X-ray photon (a type of electromagnetic radiation) and/or Auger electrons. The atom moves into an excited state with the inner shell missing an electron. By changing the number of protons, electron capture transforms the nuclide into a new element. Since the proton is changed to a neutron, the number of neutrons increases by 1, the number of protons decreases by 1, and the atomic mass number remains unchanged. In this case, one of the orbital electrons, usually from the K or L electron shell ( K-electron capture, also K-capture, or L-electron capture, L-capture), is captured by a proton in the nucleus, forming a neutron and a neutrino. For example, Rubidium-83 will decay to Krypton-83 solely by electron capture (the energy difference is about 0.9 MeV). If the energy difference between the parent atom and the daughter atom is less than 1.022 MeV, positron emission is forbidden and electron capture is the sole decay mode. Electron capture (sometimes called Inverse Beta Decay) is a decay mode for isotopes that will occur when there are too many protons in the nucleus of an atom and insufficient energy to emit a positron however, it continues to be a viable decay mode for radioactive isotopes that can decay by positron emission.


 0 kommentar(er)
0 kommentar(er)
